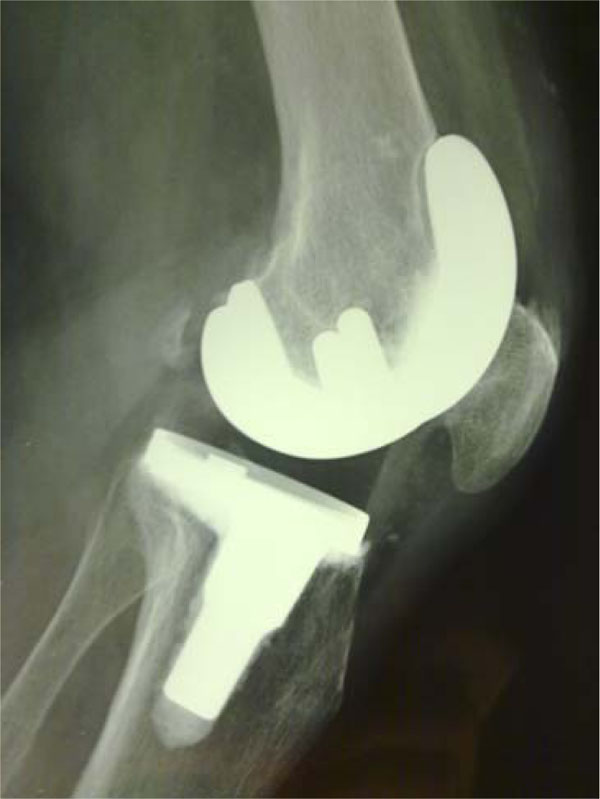
Ceramic Multigen Plus Knee With Biolox Delta Ceramic Femoral Component
The multigen plus system with a ceramic femoral component lima corporate villanova di san daniele udine italy consists of a biolox delta ceramic femoral component.
Ceramic multigen plus knee with biolox delta ceramic femoral component. Facing the brittleness and the low. The use of ceramic femoral components in total knee replacement provides tribological and allergological benefits compared to metallic components. Ceramic multigen plus knee with biolox. A national prospective duocenter study on the clinical and radiological outcome of the multigen plus total knee system with a biolox delta ceramic femoral component article january 2010 with 17.
Major prerequisite for the development of the biolox s delta ceramic knee components was the use of existing designs in order to provide the surgeon with a well known surgical technique and the same instruments as for the standard metal femoral component short learning curve. Outcomes and the reliability of the unconstrained multigen plus total knee system with a new biolox delta ceramic femoral component. All tkrs were implanted in a standardised. Cemented total knee arthroplasty was performed on 108 patients 110 knees at seven hospitals in three countries.
In case of scratching a ceramic knee surface shows just valleys unlike a metal surface which shows peaks and valleys. Biolox delta ceramic femoral component integrated in the multigen plus total knee system lima lto san daniele italy is introduced in the european market. The clinical outcome of the multigen plus knee with a biolox delta ceramic femoral component demonstrates results comparable to other studies which reported a mean postoperative 1 year hss score between 85 0 to 93 0 points 20 21. The ceramic components receive precisely executed final polishing which results in reduced surface roughness around 2 nanometers of all implant materials.
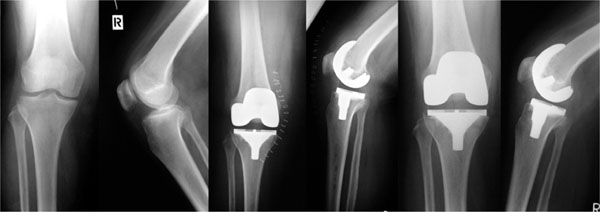
The first implantation of the multigen plus knees with the biolox delta ceramic femoral component was successfully performed in our department in december 2006. Clinical and radiological evaluations were performed preoperatively and after 3 12 and 24 months. A total of 25 tkas were included in a prospective clinical study in agreement with the inclusion and exclusion criteria after approval of the local ethical committee. The study was aimed to evaluate the clinical and radiological outcomes of the biolox delta ceramic femoral component and was classified as evidence.
The multigen plus ceramic knee is a non constrained.