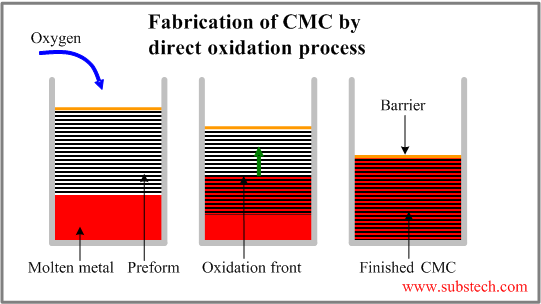
Ceramic Oxidation Reaction

Advantages of this class of fuel cells include high combined heat and power efficiency long term stability fuel flexibility low emissions and.
Ceramic oxidation reaction. This is typically done in an electric kiln. With fuel burning kilns however care must be taken to ensure that the kiln does not go into reduction until the latter part of the firing usually the last half hour to the last hour and a half. Dual 3d ceramic textile electrodes. For aln cac 2 higher oxidation rates indicate that the outward migration of ca modifies the reaction mechanisms.
With access to enough oxygen to efficiently burn your fuel you are almost exclusively producing water vapor and co2 which will cause oxidation of your ceramic surfaces. Oxidation occurs when an atom molecule or ion loses one or more electrons in a chemical reaction. Your reaction breaks down to the following for an oxidation firing using propane. Oxidation doesn t necessarily involve oxygen.
The sofc has a solid oxide or ceramic electrolyte. Originally the term was used when oxygen caused electron loss in a reaction. Linear kinetics in the range 1100 t 1200 c are followed by parabolic kinetics at higher temperatures t 1250 c. With regard to the latter behavior an activation energy of 160 kj mole could indicate the diffusion of.
The oxidation kinetic curves of the ceramic samples with compositions 1 3 at 1650 c a and the respective oxidation rates b. C3h8 5 o2 4 h2o 3 co2. When oxidation occurs the oxidation state of the chemical species increases. The modern definition is more general.
The oxidation behavior at different temperatures has been studied in terms of the variation in oxidation fraction α with time t fig. Electric kilns are naturally in an oxidation or neutral atmosphere. 2 a shows the values of α measured for the four compositions of ta hf c ternary ceramic after 30 min of exposure at 1400 c 1500 c and 1600 c together with those for the comparison samples of pure tac and hfc. A solid oxide fuel cell or sofc is an electrochemical conversion device that produces electricity directly from oxidizing a fuel.
Table 3 summarizes the values of k and n parameters obtained by approximation of the experimental data and the pearson s correlation coefficient r for each sample. By controlling of the solid content of psnb based slurry a crack free coating. This paper reports a gradient anti oxidation ceramic coating for carbon carbon c c composites which was prepared via dip coating the mixed slurry of polyborosilazane denoted as psnb a polymer precursor for sibcn ceramics zrb 2 and sic powders followed by pyrolysis and low temperature densification process. Fast kinetics for carbon oxidation reaction and oxygen reduction reaction in direct carbon fuel cells at reduced temperatures.
Fuel cells are characterized by their electrolyte material. But can also be done in gas or propane kilns. Oxidation and reduction in terms of firing schedules.